By: Editorial Staff, Date: November 28th, 2023
Delve into the intricacies of the Abbreviated New Drug Application (ANDA) pathway by gaining insights into the step-by-step process that generic manufacturers navigate to bring affordable alternatives to the market. Check out this infographic!
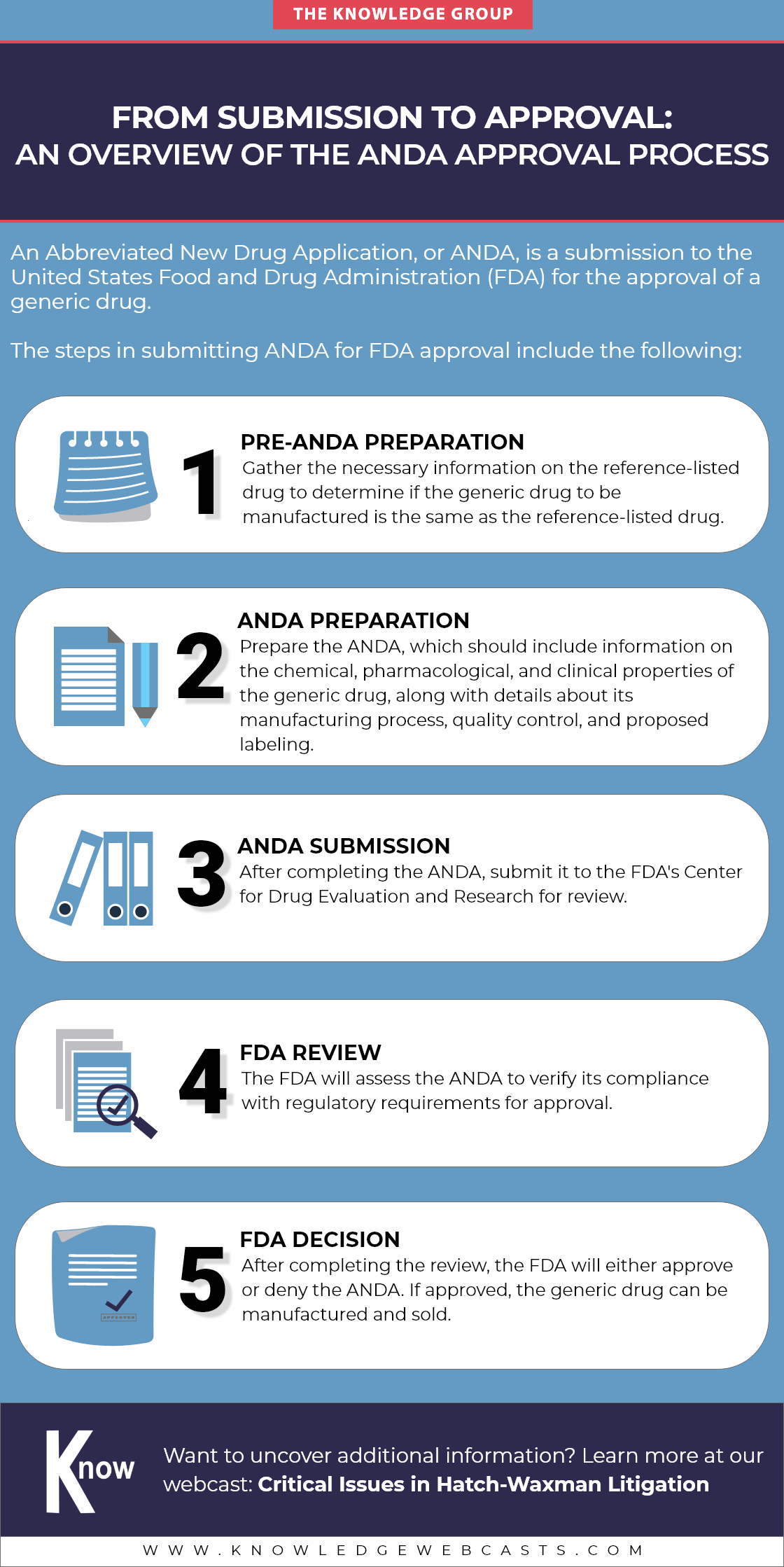
Learn more at our webcast: Critical Issues in Hatch-Waxman Litigation
Upcoming Webcasts
Breaking Down Tax Controversy: Key Issues in 2025
Class action litigation continues to be a significant force in today’s legal landscape, offering a mechanism for addressing widespread grievances and holding corporations accountable for systemic issues. However, with the complexity of handling large-scale claims, there are various legal, procedural, and strategic considerations that legal professionals must navigate. From certification to settlement, the stakes are higher, and the implications broader than in individual claims. Understanding these dynamics is crucial for lawyers, corporate counsel, and litigants alike. In this CLE webcast, Stefan Boedeker of StoneTurn Group, LLP will lead an in-depth discussion on class action litigation. He will analyze the latest trends, challenges, and strategies in managing class actions, and examine recent case law developments and their potential impact on the future of class action litigation. Additionally, he will offer insights into the ethical dilemmas and risk management strategies that arise in these cases.


