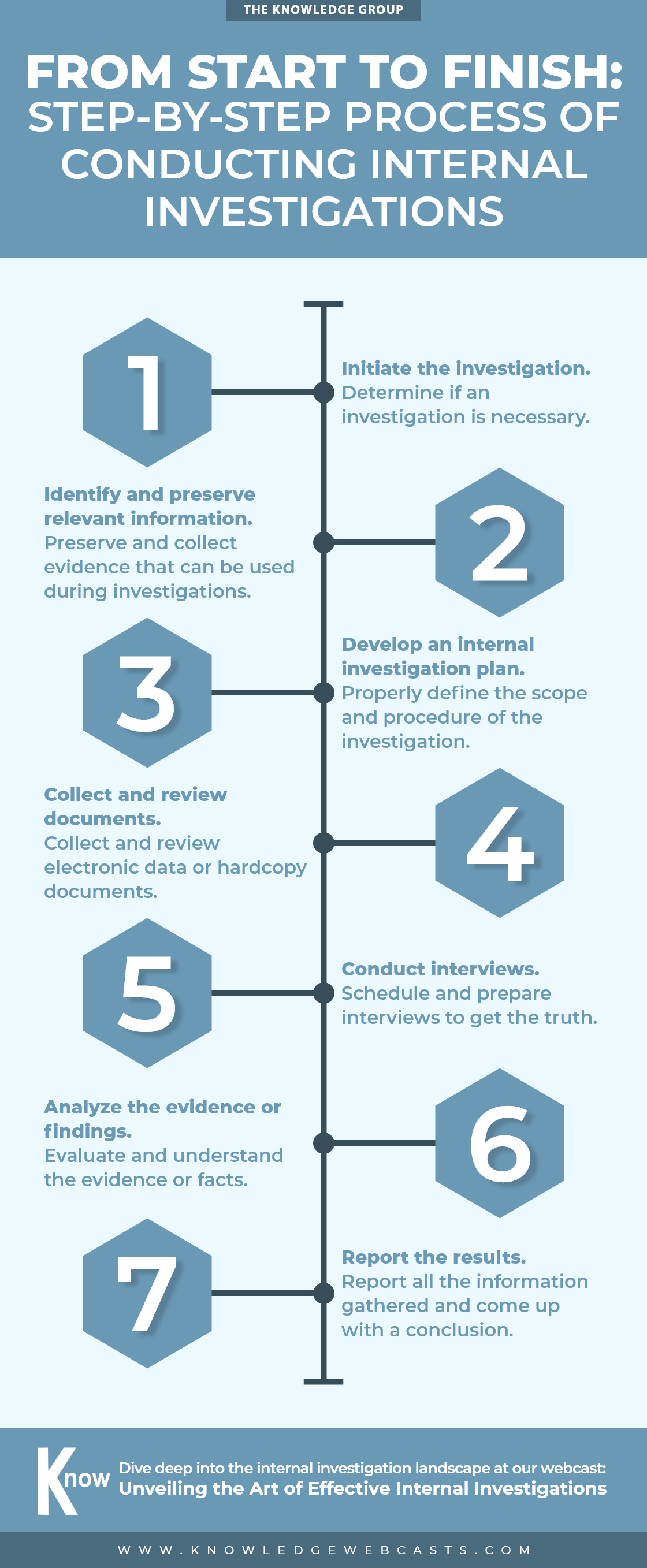
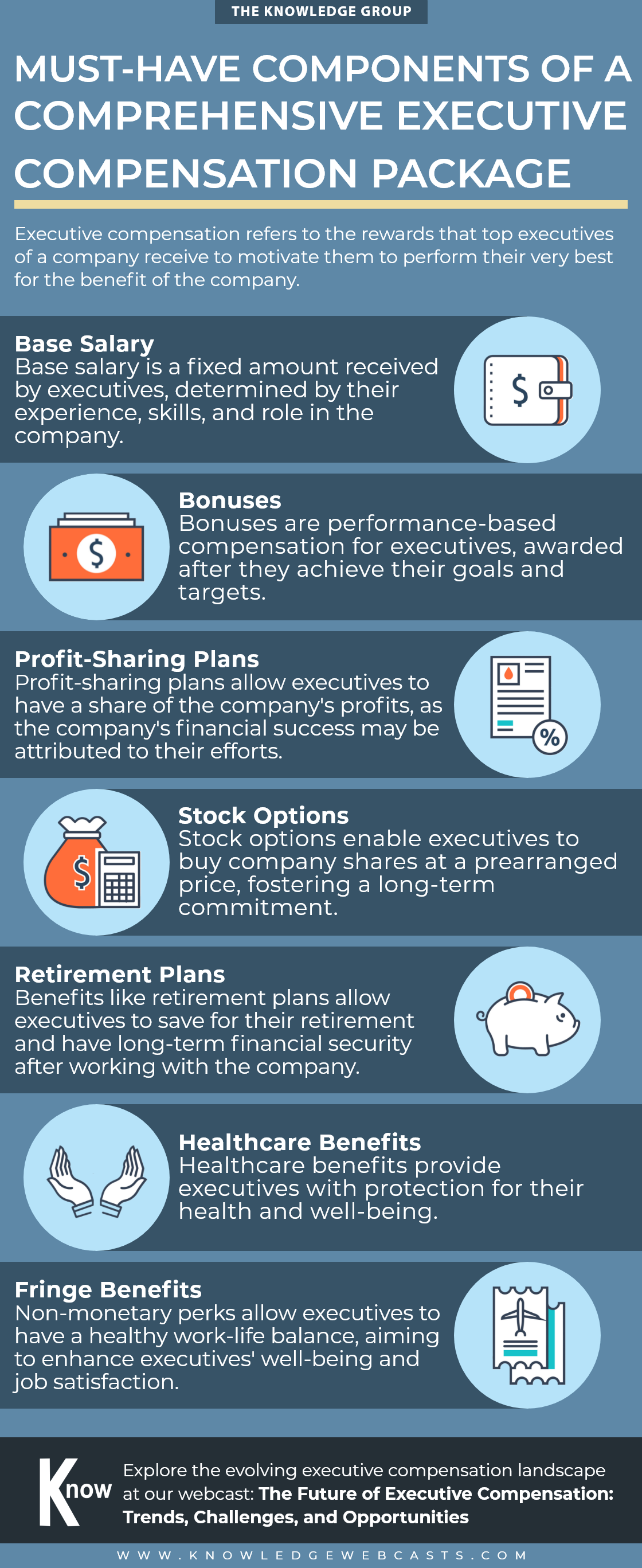
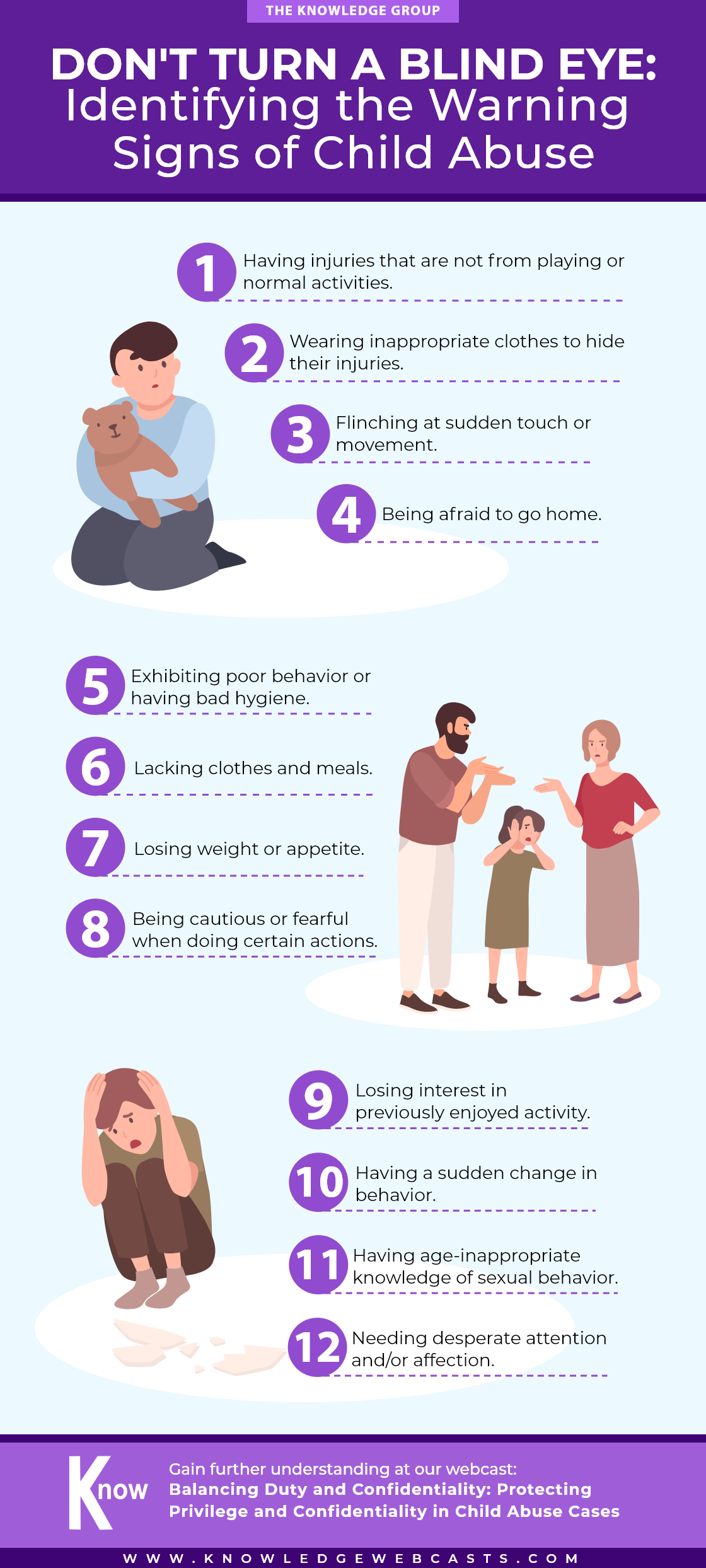
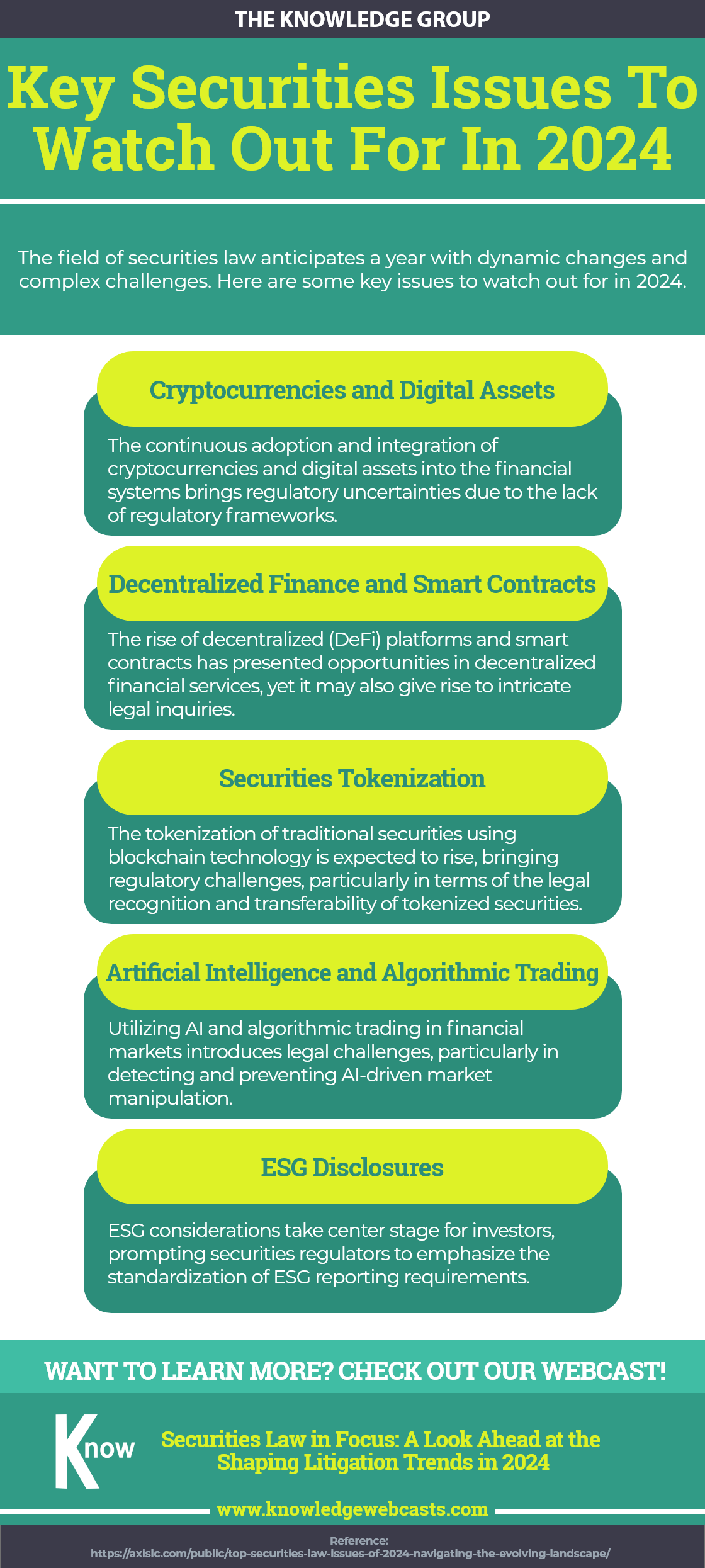
Don’t Let Your Business Sink: 10 Warning Signs to Prevent Bankruptcy
lazupardo2024-04-29T02:46:36-04:00Uncover the early indicators that could spell financial trouble for your business in this infographic. Recognize and address these critical red flags before bankruptcy becomes a reality. Gain more insights on corporate bankruptcy at our webcast: Navigating the Complex World of Corporate Bankruptcy and Reorganization Upcoming Webcasts Upcoming Webcasts back