By: Editorial Staff, Date: November 28th, 2023
Delve into the intricacies of the Abbreviated New Drug Application (ANDA) pathway by gaining insights into the step-by-step process that generic manufacturers navigate to bring affordable alternatives to the market. Check out this infographic!
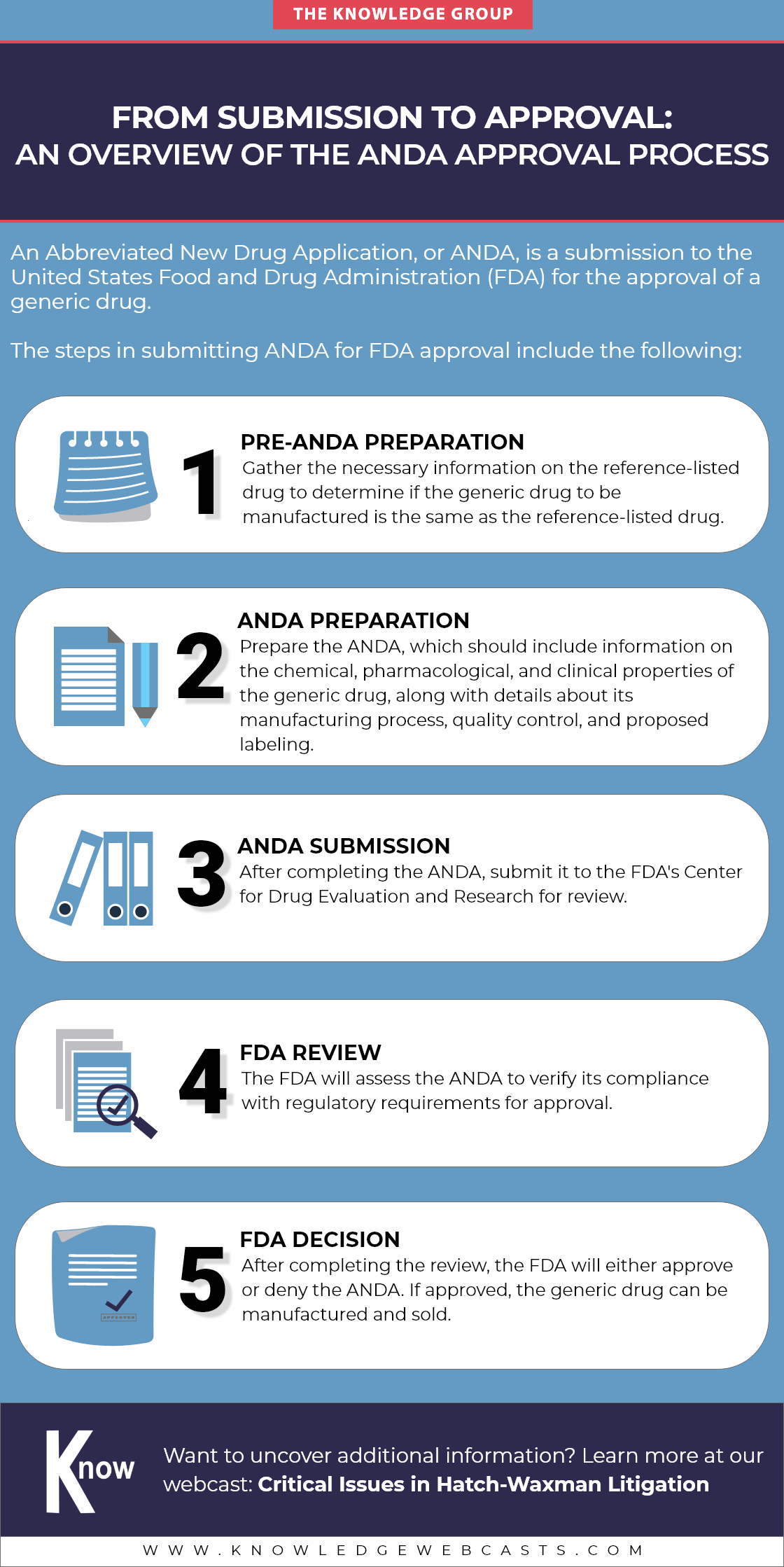
Learn more at our webcast: Critical Issues in Hatch-Waxman Litigation
Upcoming Webcasts
Ronald Allan Desiderio2025-03-24T22:26:24-04:00


